How Did The Actual Results Of Rutherford's Gold Foil Experiment
He hypothesized that the mass and positive charge of an atom are concentrated within a small part of the matter --. In his gold foil experiment rutherford shot alpha particles at very thin gold foil.

Rutherford S Atomic Model Chemistry For Non Majors
So what was Rutherfords Experiment and what.

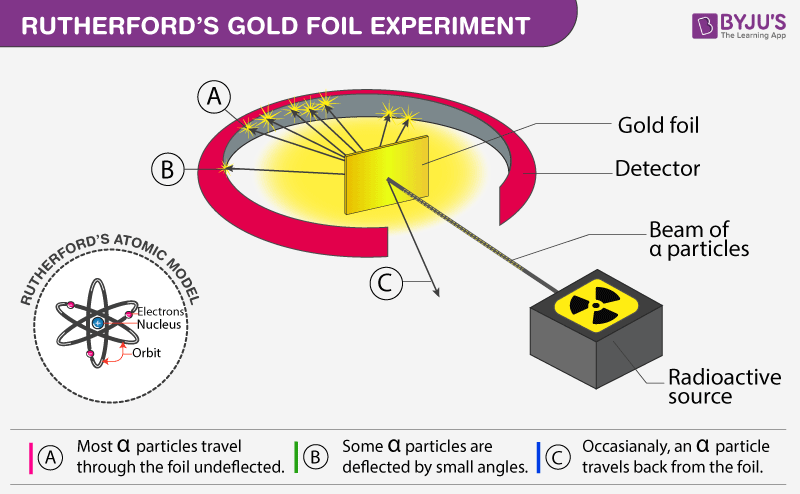
How did the actual results of rutherford's gold foil experiment. In this experiment Rutherford used Gold Foil which was extremely thin sheet not more than 1000 atoms thick. He further went on to reject the plum pudding model and developed a new atomic structure called the planetary model. They are approximately 4 times heavier than Hydrogen atoms.
Instead he found that some were deflected at very large angles. Find step-by-step Chemistry solutions and your answer to the following textbook question. How did the actual results of Rutherfords gold foil experiment differ from the results he expected.
2252009 I understand the actual experiment and the results of the gold foil experiment. Dec 12 2011 How did the results of Ernest Rutherfords goldfoil experiment showed that the atom is mostly empty space. How did the results of Ernest Rutherfords goldfoil.
How did the results of Rutherfords gold foil experiment. In this model a vastly empty atom holds a tiny nucleus at the center surrounded by a cloud of electrons. The Atomic model proposed by Ernest Rutherford was the Planetary Model and was devised on the basis of the Gold Foil Experiment.
Rutherfords Gold foil leaf experiment. All of the alpha particles passed right through the gold foil. Gold Foil was bombarded with positively charged -particles.
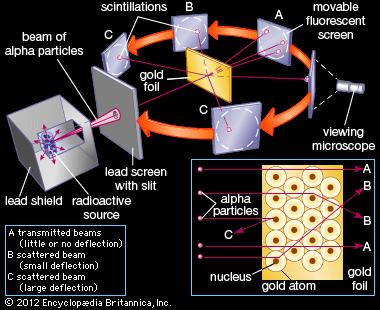
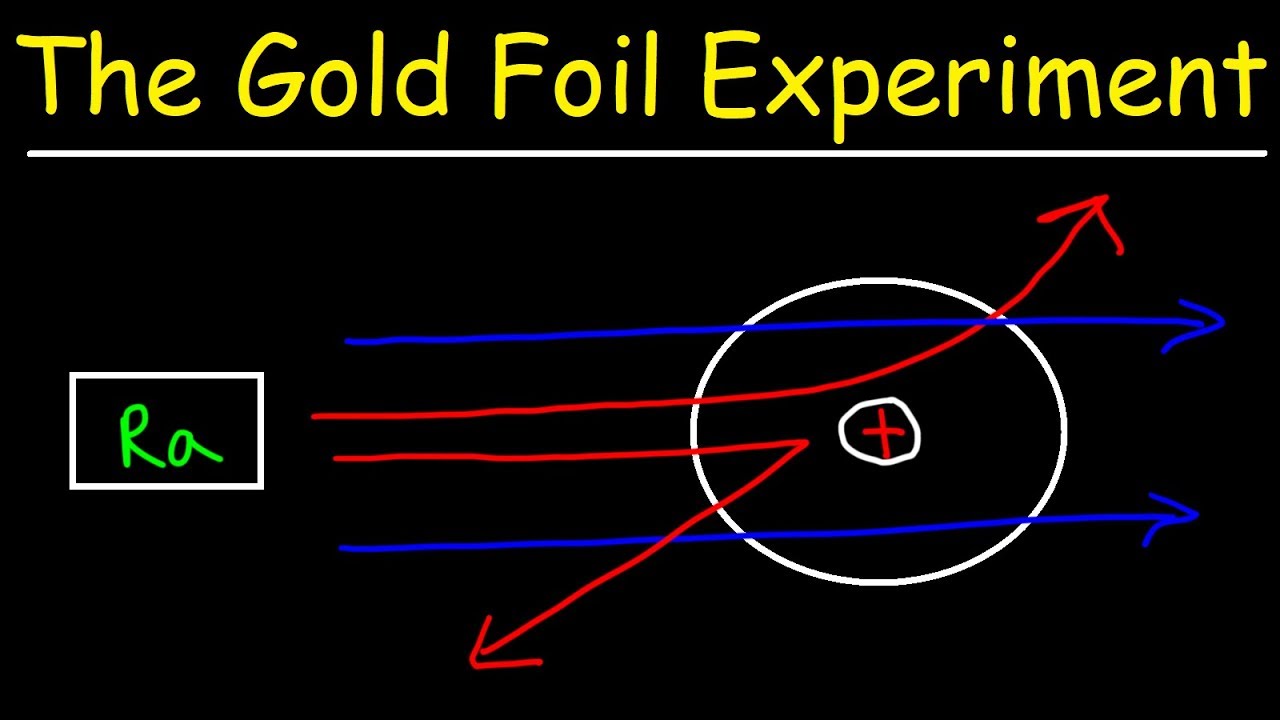
Most of the alpha particles passed through the gold foil without deflection except for a small percentage. Some of the particles were deflected and some by very large angles. As a result of his gold foil experiment Rutherfords atomic theory holds good even today.
What were the results of this experiment. Results of foil experiment if Plum Pudding model had been correct. How did the actual results of Rutherfords gold foil experiment differ from the results he expected.
Rutherford expected the a particles to be slightly deflected when they passed through a gold foil. Solved how did the actual results of rutherford s gold foil. The main conclusions of Rutherfords experiment.
If Im not mistaken his model did include a positive charge to it. What i dont understand is how the experiment tested JJ Thompsons model plum pudding of the atom. Silver and historically gold is placed in thin foils on Indian sweets.
He used -particles because they are very energetic and are heavier than other particles. Rutherford performed the Gold Foil Experiment where positively charged alpha particles were directed at a thin gold leaf suspended in a photo-sensitive chamber. Rutherfords Gold Foil Experiment was an important experiment which revealed a lot about the structure of an atom and changed the worlds perspective of an Atomic Model.
Expectation was that if the Thompson model was correct the alpha particles would be reflected away from the gold atoms because of the positive field proposed. Therefore most of the a-particles went through the gold foil without deflecting from their path. Alpha particles passing through the plum pudding model of the atom undisturbed.
Most of the space inside the atom is empty. Ernest Rutherfords famous gold foil experiment involves the scattering of alpha particles as they pass through a thin gold foilIt led to a better understan. While analyzing the results of the gold foil experiment Rutherford concluded that in order for the alpha particles to bounce back from the gold foil they had to hit something small and dense.
To prove Thomson Plum Pudding Model of the Atom is correct. Most of the alpha particles bounced back from the gold foil to the observers. There is a positive tiny part in the atom in its centre which deflects or repels the a-particles.
Expected results of Rutherfords gold foil experiment. Rutherford overturned Thomsons model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny and heavy nucleus. What change in the atomic model helped solve the problem.
This model was short lived when E. The alpha particles.
Size Of The Nucleus Rutherford Gold Foil Experiment
Experimental Evidence For The Structure Of The Atom
Plum Pudding Model Dr Atul Rana Online Tutor

Rutherford S Gold Foil Experiment

Gold Foil Experiment Overview Importance Expii
Rutherford Gold Foil Or Alpha Particles Scattering Experiment Selftution

Rutherfords Gold Leaf Experiment The Fizzics Organization
What Were Rutherford S Primary Observations In His Gold Foil Experiment And How Did He Explain Them Describe How He Modeled The Atom Socratic
Experimental Evidence For The Structure Of The Atom
Rutherford S Gold Foil Experiment Quick And Simple Youtube
Odkrycie Jadra Atomowego I Elektronow Artykul Khan Academy
Why Is The Rutherford S Of An Atom Called The Gold Foil Experiment Quora
What Happens When Alpha Particles Were Directed At A Piece Of Gold Foil Quora

Rutherford S Gold Foil Experiment





Post a Comment for "How Did The Actual Results Of Rutherford's Gold Foil Experiment"