The Gold Foil Experiment Explained
A replica of one of Geiger and Marsdens apparatus The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. They bombarded fragile sheets of gold foil with fast-moving alpha particles.

Atomic Theory Gold Foil Experiment Activity By Threefourthsme Tpt Atomic Theory Activities History Activities
They obviously had to hit something very massive and heavy analogous to a tennis ball that hits a massive concrete wall and flies back at almost the same speed.

The gold foil experiment explained. Rutherfords gold foil experiment Rutherfords alpha particle scattering experiment refers to an experiment carried out by Ernest Rutherford Hans Geiger and Ernest Marsden at the University of Manchester in the early 1900s. Rutherfords Gold Foil Experiment was an important experiment which revealed a lot about the structure of an atom and changed the worlds perspective of an Atomic Model. To operate the tutorial use the slider to increase the slit width from a range 01 to 90 nanometers.
Physicist Ernest Rutherford established the nuclear theory of the atom with his gold-foil experiment. He concluded that a tiny dense nucleus was causing the deflections. He beamed a ray of alpha particles onto a gold foil and.
Rutherford overturned Thomsons model in 1911 with his famous gold-foil experiment in which he demonstrated that the atom has a tiny massive nucleus. The gold foil experiment further showed that some -particles were reflected back to the gold foil with almost no energy loss. Khan Academy is a 501c3 nonprofit organization.
Rutherford in 1911 carried out an experiment called Gold foil experiment and could conclude the nature of an atom and the position of the protons present in the atom decisively. Rutherford in his experiment directed high energy streams of -particles from a radioactive source at a thin sheet 100 nm thickness of gold. The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom.
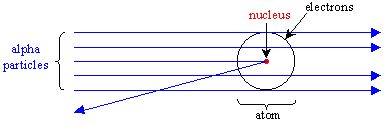
Rutherfords gold foil experiment Our mission is to provide a free world-class education to anyone anywhere. The tutorial simulates diffraction of alpha particles helium nuclei containing two positive charges by a thin foil made of gold metal. When he shot a beam of alpha particles at a sheet of gold foil a few of the particles were deflected.
When Rutherford shot particles through gold foil he found that most of the particles went through. Some of the particles though were deflected to the sides of the container around the foil and some. This chemistry video tutorial provides a basic introduction into Rutherfords Gold Foil Experiment.
Five years earlier Rutherford had noticed that alpha particles beamed through a hole onto a photographic plate. So think of the model as a spherical Christmas cake. Encyclopdia Britannica Inc.
The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre. The gold foil experiment showed that there is a nucleus by how the positively charged particles were deflected and changed course by the foil. It showed that the atom is made of mostly empty space because most particles went through the foil.
In Bohrs model the orbits of the electrons were explained by quantum mechanics. Rutherfords conducted an experiment by bombarding a thin sheet of gold with -particles and then studied the trajectory of these particles after their interaction with the gold foil. Opposite the gold foil is a zinc sulfide screen that emits a flash of light when struck by an alpha particle.
The Atomic model proposed by Ernest Rutherford was the Planetary Model and was devised on the basis of the Gold Foil Experiment. A plum pudding was a Christmas cake studded with raisins plums. He also proposed the position and behaviour of electrons.
Prior to the groundbreaking gold foil experiment Rutherford was granted the Nobel Prize for other key contributions in the field of chemistry. Some scattered in various directions and a few were even deflected back towards the source. Rutherfords gold foil experiment led to the discovery that most of an atoms mass is located in a dense region now called the nucleus.

Rutherford Gold Foil Experiment Backstage Science Youtube Experiments Gold Foil Foil

Atomic Theory Ii Chemistry Visionlearning Atomic Theory Vintage Scrapbook Paper Atom Model

Simple Solar Cooker Solar Cooker Solar Cooking Solar
Visionlearning Com Atomic Theory Atom Theories

H2 Covalent Bond Chart Potential Energy Covalent Bonding Chemistry

Rutherford S Atomic Model Experiments Plum Pudding Model Hypothesis

How It Works Atoms Atom Atomic Theory Fun Science

This Is The Plum Pudding Model It Was Developed By Jj Thompson He Proposed This Because Of The Cat Plum Pudding Model Interesting Science Facts Atomic Theory

Atomic Theory I Chemistry Visionlearning Atomic Theory Chemistry Atomic Structure

The Gold Foil Experiment Rutherford Youtube Video Monday December 3 2018 Gold Foil Experiments Foil

Atomic Theory Atomic Theory Atom Democritus Model

Nuclear Fission Gcse Science Lise Meitner Math

Rutherford S Famous Gold Foil Experiment Highlighting That Atoms Are Mostly Empty Space With A Central Dense Positively Charged Nucleus Fisica

The Gold Foil Experiment Rutherford Youtube Video Monday December 3 2018 Gold Foil Experiments Foil

The Rutherford Model Rutherford Experiment Experiments Rutherford

Rutherford Model Definition Facts Atomic Structure Ernest Rutherford Atomic Theory

Physics Nuclear Fission Explained U235 1 Neutron U236 Ba56 Kr36 3 Neutrons Physics Apologia Physical Science Nuclear Physics

Rutherford S Famous Gold Foil Experiment Highlighting That Atoms Are Mostly Empty Space With A Central Dense Positively Charged Nucleus Fisica

Power Calculation Additional Science Science Revision Energy Transfer
Post a Comment for "The Gold Foil Experiment Explained"